From static to dynamic: A chip that lets immune cells truly interact with living tissues
GA, UNITED STATES, February 4, 2026 /EINPresswire.com/ -- Predicting how circulating immune cells interact with human tissues remains a major challenge in preclinical drug testing. Traditional static culture systems often allow suspended cells to settle, creating artificial and non-physiological interactions. A newly developed microfluidic platform addresses this limitation by keeping immune cells continuously recirculating while interacting with three-dimensional tissue models. The system enables long-term co-culture of suspension cells and microtissues under controlled flow conditions, closely resembling physiological circulation. By preventing sedimentation and promoting repeated cell–tissue encounters, the platform provides a more realistic environment to study immune responses and therapeutic effects. This approach offers a powerful tool for improving the reliability of in vitro models used in drug discovery and immunotherapy research.
Advanced in vitro models such as organ-on-chip systems aim to replicate human physiology more accurately than conventional cell cultures. However, most existing platforms struggle to incorporate circulating cells like immune cells or circulating tumor cells. In static systems, these cells tend to settle by gravity, leading to non-specific contact with tissues and distorted experimental outcomes. Even perfused systems often rely on pumps or tubing, which introduce mechanical stress, complexity, and cell loss. As immune-based therapies and large biomolecules become increasingly important in medicine, there is a growing need for test platforms that maintain suspended cells in motion while enabling sustained, physiologically relevant interactions with tissues. Based on these challenges, it is necessary to develop a dynamic co-culture system that supports long-term circulation and realistic cell–tissue interactions.
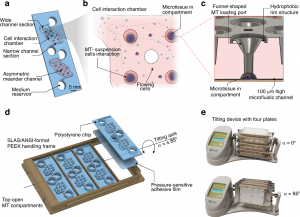
Researchers from ETH Zurich and InSphero AG reported a new microfluidic co-culture platform in Microsystems & Nanoengineering, published in 2025. The study introduces a gravity-driven chip that enables immune cells to continuously circulate while interacting with multiple three-dimensional microtissues. Using a simple tilting mechanism instead of pumps, the system maintains suspension cells for over six days without sedimentation. The platform was validated using primary human immune cells and diverse tissue spheroids, demonstrating its potential for studying immune responses and evaluating antibody-based cancer therapies under more realistic conditions.
At the core of the platform is a modular microfluidic chip featuring a central interaction chamber surrounded by seven microtissue compartments. Periodic tilting of the chip generates gravity-driven, bidirectional flow, keeping immune cells in suspension and repeatedly guiding them past the tissue models. This design minimizes unwanted cell sedimentation and mimics the recirculation seen in the human bloodstream.
Experimental validation showed that primary human peripheral blood mononuclear cells remained viable and functionally stable for at least six days under continuous flow. Compared with static cultures, perfused immune cells displayed more physiologically relevant activation patterns and avoided artificial background activation. The system also supported the long-term culture of both tumor and healthy tissue spheroids, with improved growth, metabolic activity, and functional readouts under perfusion.
As a proof of concept, the researchers modeled antibody-dependent cellular cytotoxicity using patient-derived pediatric brain tumor microtissues and immune cells. Continuous circulation allowed immune cells to infiltrate tumor spheroids and induce apoptosis when combined with therapeutic antibodies, an effect that was difficult to observe in static systems. These results highlight how dynamic flow conditions can significantly enhance the predictive power of in vitro immunotherapy assays.
"Many current in vitro assays underestimate the importance of circulation," the authors note. "Immune cells in the human body are constantly moving, and forcing them into static environments fundamentally changes how they behave." By keeping cells in motion, the platform allows interactions to occur through repeated encounters rather than artificial sedimentation. According to the researchers, this shift brings in vitro experiments closer to real physiology and opens new opportunities to study immune-mediated mechanisms that were previously difficult to capture using conventional culture methods.
The new platform could significantly improve preclinical testing of immunotherapies, including antibody-based drugs and cell-based treatments. By offering a dynamic, human-relevant alternative to static cultures and certain animal models, it may help researchers better predict treatment efficacy and toxicity before clinical trials. Beyond cancer research, the system could be adapted to study circulating tumor cells, immune surveillance, and tissue-specific targeting under controlled flow conditions. Its pump-free design and scalability also make it suitable for higher-throughput screening, supporting more reliable and efficient development of next-generation therapeutics.
References
DOI
10.1038/s41378-025-01028-9
Original Source URL
https://doi.org/10.1038/s41378-025-01028-9
Funding Information
The project was financially supported by the Innosuisse grant 38880.1 IP-LS. and by the “Personalized Health and Related Technologies (PHRT)” of the ETH Domain (Project #2021-351). We also thank the Swiss to Cure and Isabella Kerr Foundation for their support of DMG research.
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.